(I’ve added some late postscripts at the end of this post.)
The question before us is whether the U.S. Fish and Wildlife Service (FWS) should remove the gray wolf in the U.S. from the list of endangered species. As we discussed in previous posts, wolves in Alaska, in the Northern Rocky Mountain distinct population segment, and in the Western Great Lakes distinct population segment are already off the list. Mexican gray wolves in Arizona and New Mexico and red wolves in North Carolina will continue to be protected.
I concluded in an earlier post that delisting the gray wolf would not affect present wolf populations greatly. Almost all known wolf packs are either already off the list or, in the case of Mexican and red wolves, will continue to be protected regardless. Delisting the gray wolf may prevent new packs from establishing themselves in new areas, because it will be legal to shoot wolves as they travel from established population centers into these new areas. The U.S. Fish and Wildlife Service (FWS) is in effect deciding whether to allow wolves to spread naturally or to hinder that spread. Suzanne Stone, Northern Rockies representative for the Defenders of Wildlife, an environmental organization, voiced these fears as quoted in a story by Northwest Public Radio:
Stone: “With the federal delisting that they are talking about – pulling federal protections from wolves before there are even wolves on the ground in some of the best wolf habitat in the western United States.”
Stone’s offices are in Idaho, where the wolf population last year was nearly 700. She is concerned that wolf hunting in Idaho and Wyoming will keep them from migrating into neighboring states of Utah and Colorado, or expanding their small populations in Oregon and Washington. Those two Northwest states had a combined population of about 100 wolves.1
Most environmental groups are opposed to delisting, and I respect that. But there is an argument for not fighting delisting. If delisting will not destabilize present wolf populations, and I don’t think it will, then perhaps fighting delisting is not the best use of resources, besides making enemies we don’t need to make. There is also another aspect we need to be concerned about. Despite our disagreements with the Obama Administration, it has been far more sympathetic to environmental concerns than any Republican administration would have been. Why do environmental organizations insist on taking the Administration to court when they could be striving together with the government to reach common goals? Aren’t we frittering away historic opportunities to work with the Federal Government, opportunities that if a Republican president is ever elected are not likely to return for four or maybe eight years, if then?
I think environmental groups should cut a deal with this Administration. They should agree to drop all lawsuits opposing wolf delisting. In return, the Administration should commit itself to the goal of establishing five new major wolf population centers and a total population in the 48 states of 20,000 wolves by 2030 (I’m just suggesting those numbers; maybe there are better ones.). Next, everyone needs to work closely with states to make this happen. I suggest that the Federal Government agree to cede Federal lands to states in return for their cooperation.
Several government agencies must get on board for this happen. Most important are the Fish and Wildife Service and the Department of Agriculture’s APHIS Wildlife Services, responsible for predator control. The White House is essential to provide political backing in what is sure to be a controversial initiative. State governments must be brought into the process (although whether this is possible depends a great deal on local political conditions).
Environmental groups are critical as well — they provide the impetus, the will, the enthusiasm, the energy for all this to happen. They can organize and energize the popular support necessary to move the project forward and provide cover to politicians to get the job done.
We environmentalists must be realistic. We may not be able to get everything we want. But we can accomplish much more working together with the government than we can constantly fighting it in court. With all the legal action being pursued in the past ten years, has a single new wolf population center been established?
(Of course, the Federal Government can give a flat no, in which case my argument goes out the window. If then environmental groups think they can win in court, then perhaps they should continue suing. But they need to perform a careful cost-benefit analysis to avoid wasting their time, resources, and their supporters’ money.)
Where can we establish new wolf population centers? This is the map put out by Defenders of Wildlife that I had published in a previous post2:
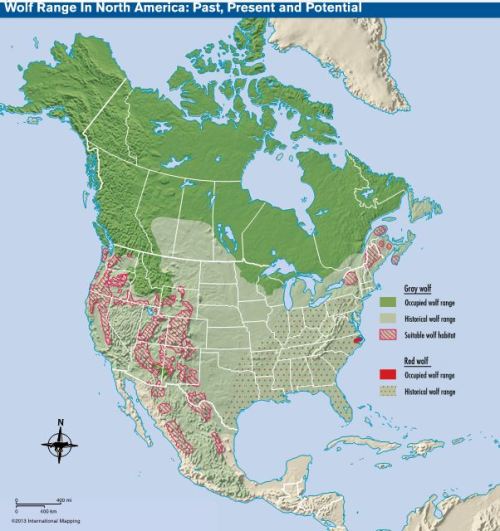
Note the red-striped areas on the map in the west and northeast which indicate suitable wolf habitat. Wolves can be introduced to California, Nevada, Utah, Colorado, Texas, New York, and Maine, and new ranges can be established in Washington State, Oregon, Wyoming, Arizona, and New Mexico. The most important criteria in choosing new areas is where wolves can make a significant contribution to biodiversity. If introducing wolves will enable more species of animals and plants to thrive, then the initiative is worth doing, otherwise what’s the point? After that, we need to consider the potential for conflict with humans and how we can alleviate such conflict. What’s the human population of the area, how many pets do they have, how much livestock are they raising? Last but not least, we need to consider the amount of political opposition we are likely to encounter, and we are likely to encounter a great deal.
Perhaps the Wyoming model may best alleviate conflicts with humans, despite my criticism of Wyoming in a previous post. Wyoming has zoned the state into wolf habitat and non-wolf habitat. Wolves in wolf habitat can only be killed during hunting season in the fall by licensed hunters, whereas wolves elsewhere may be shot by anyone at anytime3. I assume people know enough not to grow livestock in wolf habitat (and if they do, they must be prepared to accept the consequences), and wolves won’t last long enough to attack livestock in non-wolf habitat. Now we can argue about the propriety of the amount of land set aside for wolves (about 16.5% of the area of the entire state, leaving 83.5% of Wyoming off limits to wolves4), but I think the model can work to bring problems with wolves down to a minimum. At the time of this post, environmental organizations have sued the U.S. Fish and Wildlife Service over its delisting of the wolf in Wyoming, demanding that wolves in Wyoming be returned to Federal protection4. Whether Wyoming can successfully manage its wolf population without Federal intervention, only time will tell.
Living in New York State (Brooklyn, actually), I would like to see the wolf return to Adirondacks Park. Adirondacks Park is huge, over 6 million acres, and occupies a good part of northern New York. It is hard to see how there is not enough room in this large area for several wolf packs. Here is a map taken from Wikipedia showing the location of Adirondacks Park with New York State5.
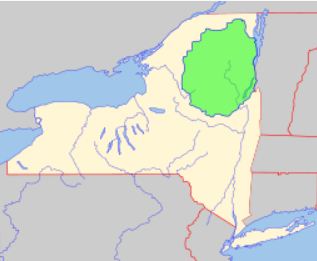
There has been talk of returning the wolf to the Adirondacks before, although not recently. In December 1999, the Adirondack Citizens Advisory Committee released a report on the feasibility of the reintroduction of the wolf to the Adirondacks. The report concluded that although Adirondacks Park could support a wolf population, human development would threaten the wolves’ long term prospects6. I don’t think that’s a good enough reason not to go forward. We need to experiment; the only way we can know if wolves can thrive in the Adirondacks is to try. I suspect that wolves, who as a species have a well-deserved reputation for being tremendously resilient7, will be able to withstand human development and will thrive. (New York State’s constitution does guarantee that the Adirondacks will be forever wild8). In fact, there is an organization called the Northeast Ecological Recovery Society that is dedicated, among other things, to the restoration of the wolf to the Adirondacks. You can read what they have to say about this on their website by clicking here. On there website, be sure to click on their essays “The Missing Piece” and “How Deer Benefit.”
There are two groups who have been most outspoken in their opposition to further wolf recovery, livestock producers and hunters, who resent the loss of wild game to wolves. I can particularly sympathize with individual ranchers who feel anxious and threatened by the presence of wolves (see the video on the oregonwolfeducation.org website by clicking here). These people should have the right to shoot any wolf that threatens their property, including any wolf that strays within 1000 yards of any livestock herd. Furthermore, if wildlife scientists determine that the amount of wild game in a particular area is not large enough to support the resident wolf population and that these wolves will likely turn on livestock as an alternative, government should cull the wolves so that doesn’t happen.
But what I object to is livestock producers having any say in how wilderness areas are managed. Wilderness areas should be managed for the benefit of the natural ecosystem that exists within it and not to protect any narrow human economic interest. To do otherwise is to defeat the whole purpose of having a wilderness. Adding wolves to wilderness areas enhances the ecosystem and brings it closer to the state it was before humans came. If wolves leave the wilderness and cause problems, that will need to be dealt with, but that is no reason not to reintroduce wolves in the first place.
Now for the hunters. I have no public objection to hunting, although I would never do it myself. Furthermore, I have no problem with setting aside lands as hunting preserves, semi-wild places which are managed to maximize numbers of wild game and exclude predators. But true wilderness areas should be kept wild and not managed to please humans. By all means, let people hunt in the wilderness, but they must do it on nature’s terms, not theirs. That means they must learn to compete fairly with natural predators who have as much right to hunt there as they do.
Hunters complain that wolves are decimating elk and deer herds, but that might not be true at all. True, wolves do reduce the elk and deer population, but that’s fine as long as the population remains at sustainable levels (however, if the population can no longer sustain the wolves and the wolves are forced to leave the wilderness to attack livestock, then culling the wolves becomes necessary). In fact, lower elk and deer population numbers can be better for the ecosystem as a whole, as has been shown in Yellowstone9. There is no obligation to keep elk and deer numbers high just to please human hunters.
To illustrate the ludicrousness of killing wolves in wilderness areas just to maximize game animals and please human hunters, I’ll give you a argument in the economic sphere. Microsoft completes with Apple, would it have been OK for Bill Gates to shoot Steven Jobs? Would it be alright for Ford to firebomb General Motors production plants? How about Walmart burning down Target stores?
Of course these are crimes. We even have laws prohibiting practices that are deemed monopolistic or anticompetitive even if they are not violent. Similarly, we should not penalize the wolf for outcompeting humans. If humans want to hunt game, let them outcompete the wolf. Let them learn to track elk and deer and find them before the wolves do. When you hunt in a wilderness area, don’t expect game to be served to you on a silver platter, work for it, just like the wolves do! Hunt, but compete fairly. Do not try to establish species monopolies.
These are only my ideas. Defenders of Wildlife has put together an excellent plan for the recovery of wolves that does a much better job than I have at making the case for wolves, entitled Places for Wolves: A Blueprint for Wolf Restoration and Recovery in the Lower 48 States which you can read by clicking here.
In the meantime, we can continue to learn from the populations of wolves we already have. How do areas with wolves within a state compare with those without wolves — are they really more biologically diverse? Do they appear more ecologically robust and resilient? How well are the states managing their packs? Are they successful in minimizing wolf-human conflicts? Do the humans who live in proximity to the wolves feel comfortable or do they feel threatened? What we learn in states with wolf populations will be invaluable in returning this remarkable keystone species to its rightful place in the American landscape.
Postscripts:
It seems that the Idaho legislature proved more determined than I thought it was to decimate its wolf population. For details, see the post in my opinion blog: Wolves in Idaho.
In the May 2014 issue of Discover Magazine, there is an excellent article by Christie Wilcox, Elk Vanishing Act. Unfortunately, the article is only available online to subscribers. The article describes how biologists have show that wolves are not the main cause of decline of elk in Yellowstone National Park. While they prey on elk, their contribution is rather small. A much larger contribution are grizzlies who are turning to elk when their previous source of protein, cutthroat trout, were out-competed by the illegally introduced lake trout. Also to blame are severe droughts in the park (possibly the result of climate change) which reduced the amount of grass available to the elk. The article cites the work of biologists Scott Creel, Arthur Middleton, Shannon Barber-Meyer, and Jennifer Fortin.
Footnotes:


